EU raises its bet on blood plasma in search for COVID-19 therapy
- EU asks blood agencies to apply for funding - document
- Says emergency funds could be used for plasma collection
- Plasma being tested across world for COVID-19 treatments
BRUSSELS: The European Union wants to fast-track funding to treat COVID-19 patients with blood plasma collected from survivors, an EU document seen by Reuters shows, in a sign of the bloc's growing confidence in the experimental treatment.
The move also highlights the more assertive approach being taken by the 27-nation union in the race to find effective drugs and vaccines against the new coronavirus, after the United States scooped up several promising candidates.
The European Commission, the EU's executive arm, has invited national blood authorities to apply for possible emergency funding by July 10 to boost their collection of convalescent plasma, which is obtained from people who have recovered from COVID-19, the document seen by Reuters said.
Funds could be used to buy equipment to collect, store and test convalescent plasma, the document said, adding the money could come from the Emergency Support Instrument (ESI), a European rainy-day fund.
The use of the ESI could allow funds to be provided this year. Usually EU funding projects are planned years in advance.
Money from the 2.7-billion-euro ($3 billion) ESI has so far only been used or committed for highly sensitive issues, such as buying scarce face masks at the peak of the pandemic in Europe and advance purchase of potential COVID-19 vaccines .
Over 300 million euros have been spent and about 2 billion is pencilled in to buy possible vaccines, EU officials told Reuters. This leaves some 400 million euros available.
The use of the ESI is still being considered, the Commission noted in its document. A Commission spokesman did not immediately reply to questions on the matter.
PLASMA RUSH
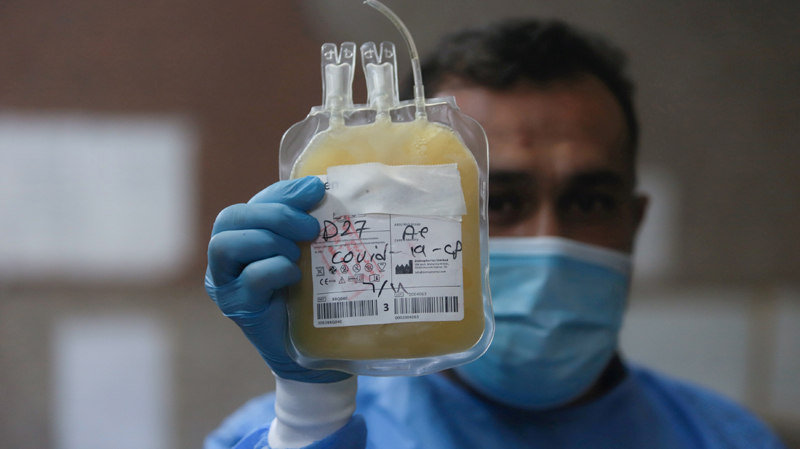
Since the beginning of the pandemic, medics across the world have been transfusing convalescent plasma into critically ill COVID-19 patients, often with positive results, although its efficacy is still under investigation.
People who survive an infectious disease like COVID-19 are left with blood plasma containing antibodies, or proteins made by the body's immune system to fight off a virus, that can be transfused into newly infected patients to try to aid recovery.
Plasma, which is the liquid component of blood, is also being tested by public authorities and companies to develop medicines against COVID-19, such as hyperimmune globulins.
Separate research is underway on its possible use to prevent COVID-19 infections, as antibodies extracted from it could be transfused to boost immunity defences of vulnerable people. That could be particularly important in the absence of a vaccine.
The Commission has already funded research on convalescent plasma, but unblocking emergency funding to promote collection would be the boldest move so far.
The EU is currently financing a project to develop a plasma-derived therapy against COVID-19 and has also set up a database to share results of treatments applied in European hospitals.
It is also working to reduce its long-standing dependency on plasma imported from the United States to manufacture critical non-COVID medicines such as immunoglobulins and medication that helps control bleeding.